Companion Diagnostics Explained: Their Critical Role in Cancer Care and Our Latest Approvals
NOW FDA APPROVED: OUR NEWEST* COMPANION DIAGNOSTIC INDICATIONS
Below are the newest* additions to our roster of companion diagnostic approvals across multiple cancers and our portfolio of FDA-approved comprehensive genomic profiling tests. Check back frequently, as new companion diagnostic indications continue to be added.
FoundationOne®CDx and FoundationOne®Liquid CDx: Recent Companion Diagnostic Approvals*
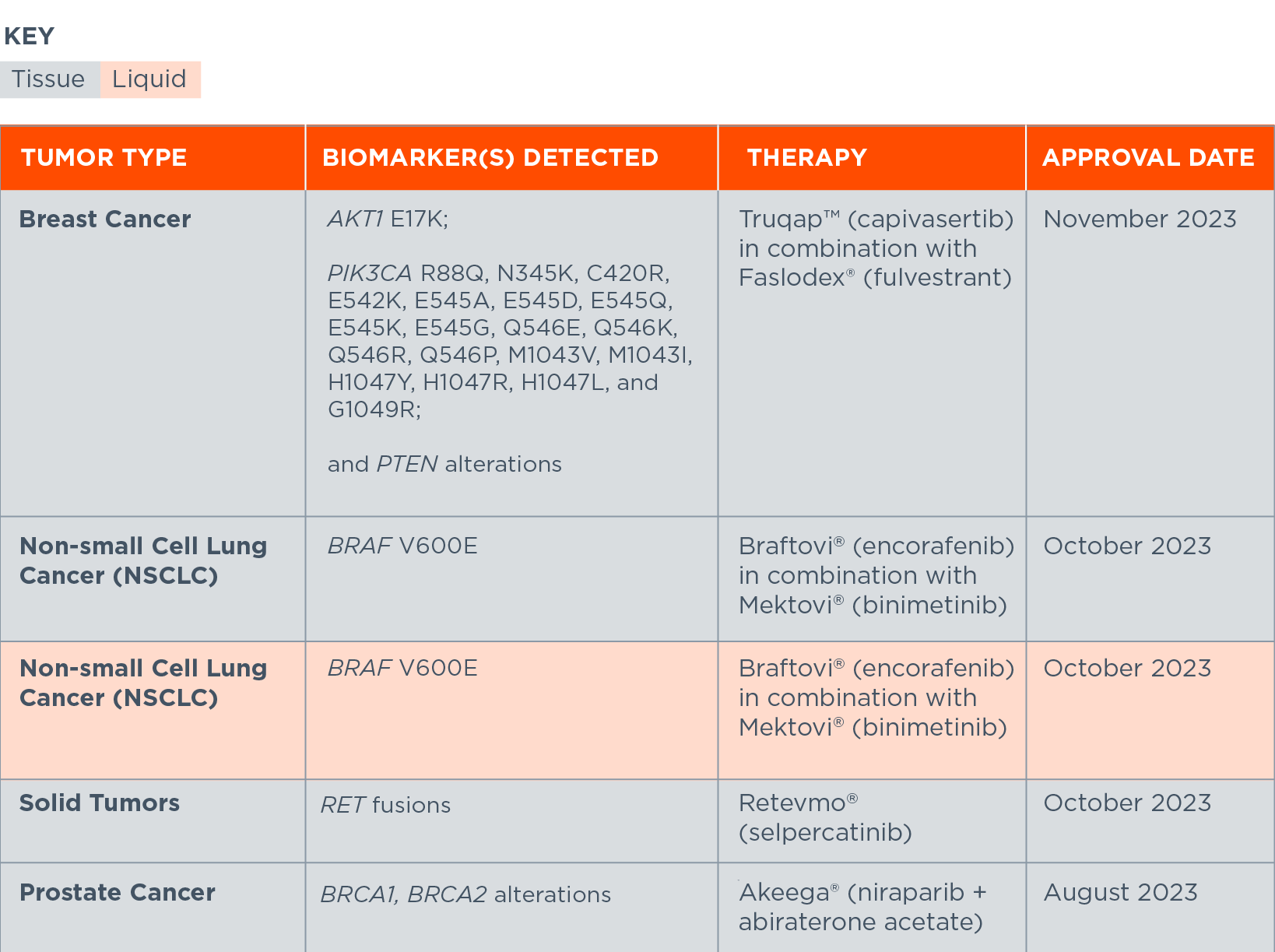
*Includes companion diagnostics approved within the last six months, organized by approval date, then alphabetically within each cancer type and FDA-approved therapy. A complete list of CDx approvals for both FoundationOne CDx and FoundationOne Liquid CDx is below.
TURNING THE VISION OF PRECISION MEDICINE INTO REALITY THROUGH THE POWER OF COMPANION DIAGNOSTICS
When oncologists are treating advanced cancer patients, they need to move quickly from insight to action. To do so, they need comprehensive, reliable information about what is driving a patient’s cancer so they can make personalized treatment decisions. We’ve forged a path forward to achieve the highest quality standards for our tests – to both improve adoption of comprehensive genomic profiling (CGP) and access to this technology in the clinic.
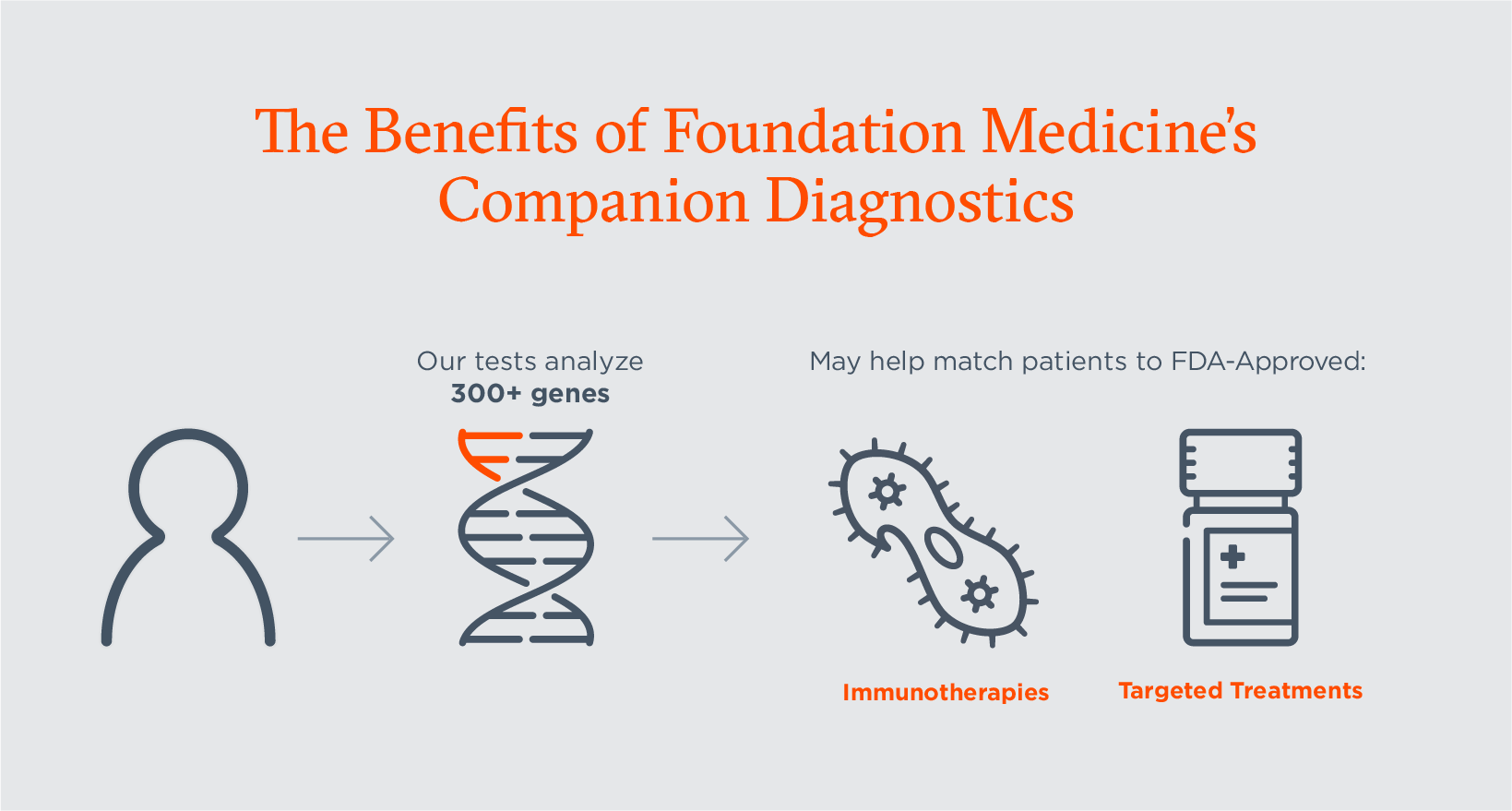
CGP helps inform decisions on available treatment options, including targeted therapies, immunotherapies, tumor-agnostic treatments and clinical trial participation, based on the unique genomic profile of a patient’s tumor. Contrary to smaller panels like hotspot or single gene tests, CGP tests deliver comprehensive information in a single test and can provide reliable information on complex genomic signatures. Foundation Medicine’s full portfolio of CGP tests and its molecular insights platform are helping improve day-to-day care for cancer patients by providing information on:
- Hundreds of relevant genes known to drive cancer, including all guideline-recommended genes in solid tumors and additional genes that may be of relevance for targeted therapies in the future.
- Interpretive content that can be used for patient management, including clinical trial options, in accordance with professional guidelines in oncology.
- When a patient may benefit from FDA-approved therapies for which our tests are FDA-approved companion diagnostics.
Companion diagnostics allow physicians to identify patients who may benefit from FDA approved treatment options. And, as the number of targeted oncology therapy indications and approvals in oncology grow rapidly, companion diagnostics provide information that is critical for the safe and effective use of targeted therapies.
FoundationOne CDx and FoundationOne Liquid CDx have been approved with several companion diagnostic claims and will serve as platforms for further companion diagnostic development, meaning that through supplemental Premarket Approval Applications (PMAs), companion diagnostic claims for additional FDA approved therapies and immunotherapies can be added.
Read on to learn more about Foundation Medicine’s tests and the important role that CGP and companion diagnostics play in precision cancer care.
COMPANION DIAGNOSTICS 101
What is a companion diagnostic?
- Companion diagnostics provide information that is critical for the safe and effective use of targeted therapies.
- An FDA approved companion diagnostic test is used to determine which FDA-approved treatment options a patient may benefit from based on the genomic alterations within their tumor(s).
How do comprehensive genomic tests and companion diagnostics help inform cancer care?
- A comprehensive approach to genomic testing provides a complete picture of a patient’s tumor and offers insights to clinicians about the treatment protocols that may benefit patients, including targeted therapies, immunotherapies, tumor-agnostic treatments and clinical trials.
- A companion diagnostic, or CDx, informs the use of personalized treatment options for advanced cancer patients by identifying FDA-approved treatment options that may be appropriate based on the unique drivers of their individual cancer.[i]
- These matched treatments are listed on the first page of Foundation Medicine’s genomic reports.
- Foundation Medicine’s companion diagnostic tests:
- Offer rigorously-validated and high-quality genomic insights;
- Can be performed using either tumor tissue or blood;
- Cover all four types of alterations, including oncogenic fusions;
- Analyze more than 300 cancer related genes to inform the use of precision cancer treatments across all solid tumors; and
- Can identify complex genomic signatures.
Why is FDA approval of diagnostic tests important?
- A diagnostic test approval by the U.S. Food and Drug Administration (FDA) means it has been analytically and clinically validated, and is safe and effective for its intended use.
- Foundation Medicine offers the only FDA-approved portfolio of comprehensive genomic profiling tests for all solid tumors.
Why is a broad approach to genomic testing needed?
- Tumor testing aimed at uncovering alterations in a single gene or a small number of genes (or “hot spot testing”) provides limited results.
- This approach can miss actionable alterations and therefore, opportunities to match patients to an appropriate therapy. This approach can cost valuable time, can require multiple specimen samples and may not test for all potentially actionable alterations.[ii] This can lead to treatment delays or insufficient information to guide next steps for treatment.
- With the rapid growth of precision medicine and more than 50% of pipeline therapies projected to ultimately have an associated biomarker, broad genomic testing – which analyzes many genes in one test to determine suitability for many treatments and helps inform a timely start to care – could make a significant impact on accelerating the treatment landscape.[iii]
- FoundationOne CDx and FoundationOne Liquid CDx are approved as companion diagnostic for multiple therapies, allowing more patients to potentially match to a range of FDA approved therapies based on alterations that may be found.
- A comprehensive approach to testing also keeps oncologists up to date with the most recent treatment options for their patients. These tests serve as platforms for future companion diagnostic development and Foundation Medicine notifies existing customers when certain therapies are approved and emerging biomarkers become relevant to existing patient profiles.
Do Foundation Medicine’s tests identify mutations that do not have an associated companion diagnostic?
- Foundation Medicine’s liquid- and tissue-based tests analyze more than 300 cancer related genes, some of which do not yet have approved therapies.
- As a professional service, which is not reviewed or approved by the FDA, Foundation Medicine’s tests identify FDA-approved therapies that may have clinical benefit for the patient based on their molecular profile, but for which are not FDA-approved companion diagnostics.
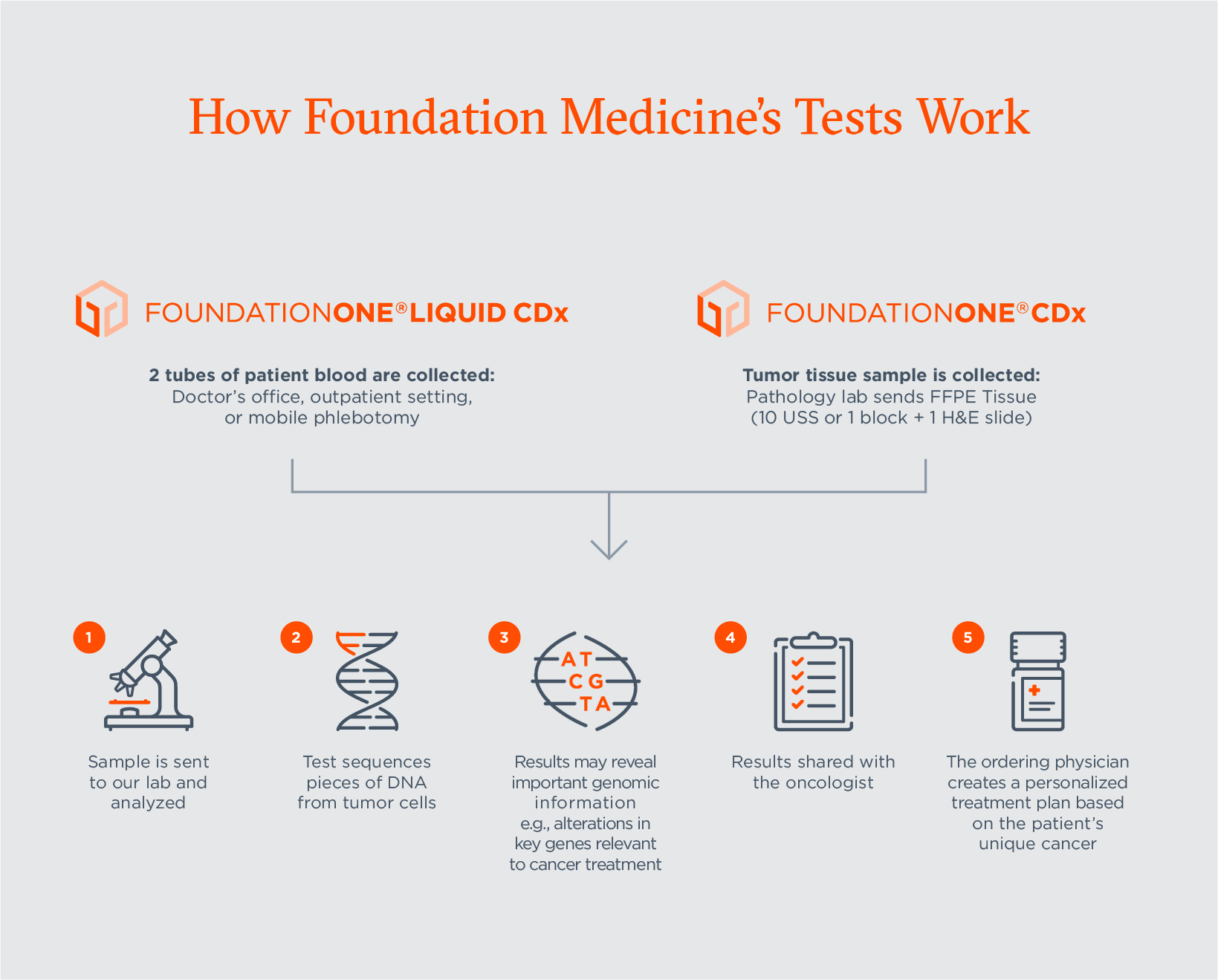
What is the difference between FoundationMedicine’s platform tests, FoundationOne®CDx and FoundationOne®Liquid CDx?
Foundation Medicine is committed to providing oncologists with the tools that they need to make treatment decisions for their patients. We recognize that every patient’s situation is unique, and that there are varied scenarios where one of our tests will make more sense than the other. For example, if tumor tissue is readily available, tissue testing may be the most appropriate means of genomic sequencing. But for another patient who may be too sick to undergo another surgery in order to acquire tissue, the option for liquid biopsy could make more sense.
With two FDA-approved tests that both detect over 300 cancer-related genes, we can provide doctors with options to consider when electing to prescribe comprehensive genomic testing. Both tests allow physicians to uncover deep genomic data that can help inform treatment decisions – whether it be through tissue or blood-based biopsies.
- Our FDA-approved platform tests include:
- FoundationOne®Liquid CDx: this liquid biopsy test – our most recently FDA-approved test – offers an important, minimally invasive comprehensive genomic testing option for patients, including those who may not have been eligible for tissue-based genomic testing because of their health status, tumor location or cancer type. See our full list of companion diagnostic claims below.
- FoundationOne®CDx: this tissue biopsy test – Foundation Medicine’s flagship test – was the first FDA-approved broad companion diagnostic for solid tumors. It is approved as a companion diagnostic to identify patients who may benefit from treatment with more than 20 therapies across multiple cancer types. See our full list of companion diagnostic claims below.
ADDITIONAL RESOURCES – LEARN MORE:
- FoundationOne®Liquid CDx Liquid Biopsy Test
- FoundationOne®CDx Tissue Biopsy Test
- Compare Our Tests: Our Testing Portfolio
- Online Ordering & Report Integration
- Genomic Testing Patient Video: The Foundation Medicine Approach
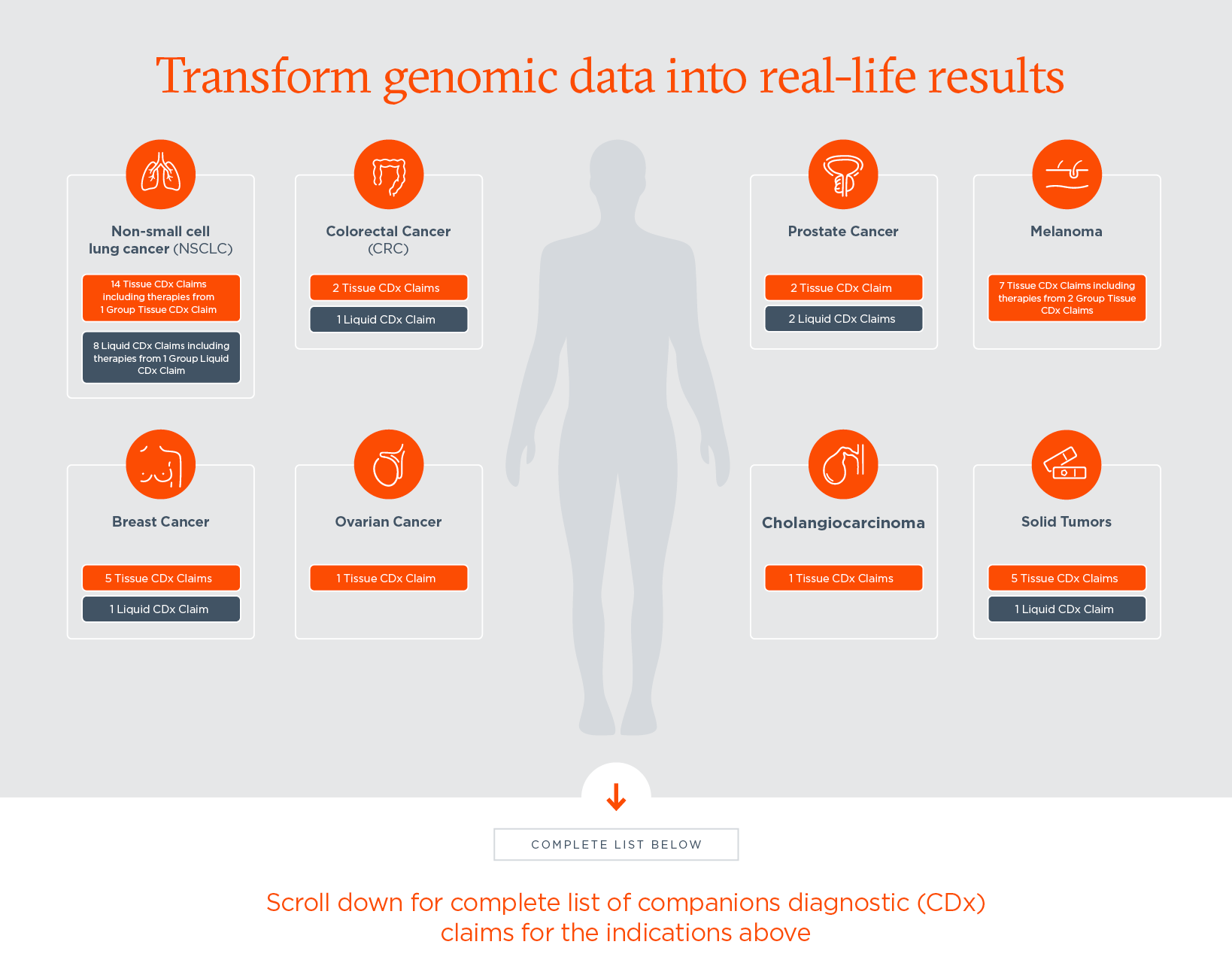
FULL LIST OF FDA-APPROVED COMPANION DIAGNOSTICS FOR FOUNDATIONONE® CDX AND FOUNDATIONONE®LIQUID CDX
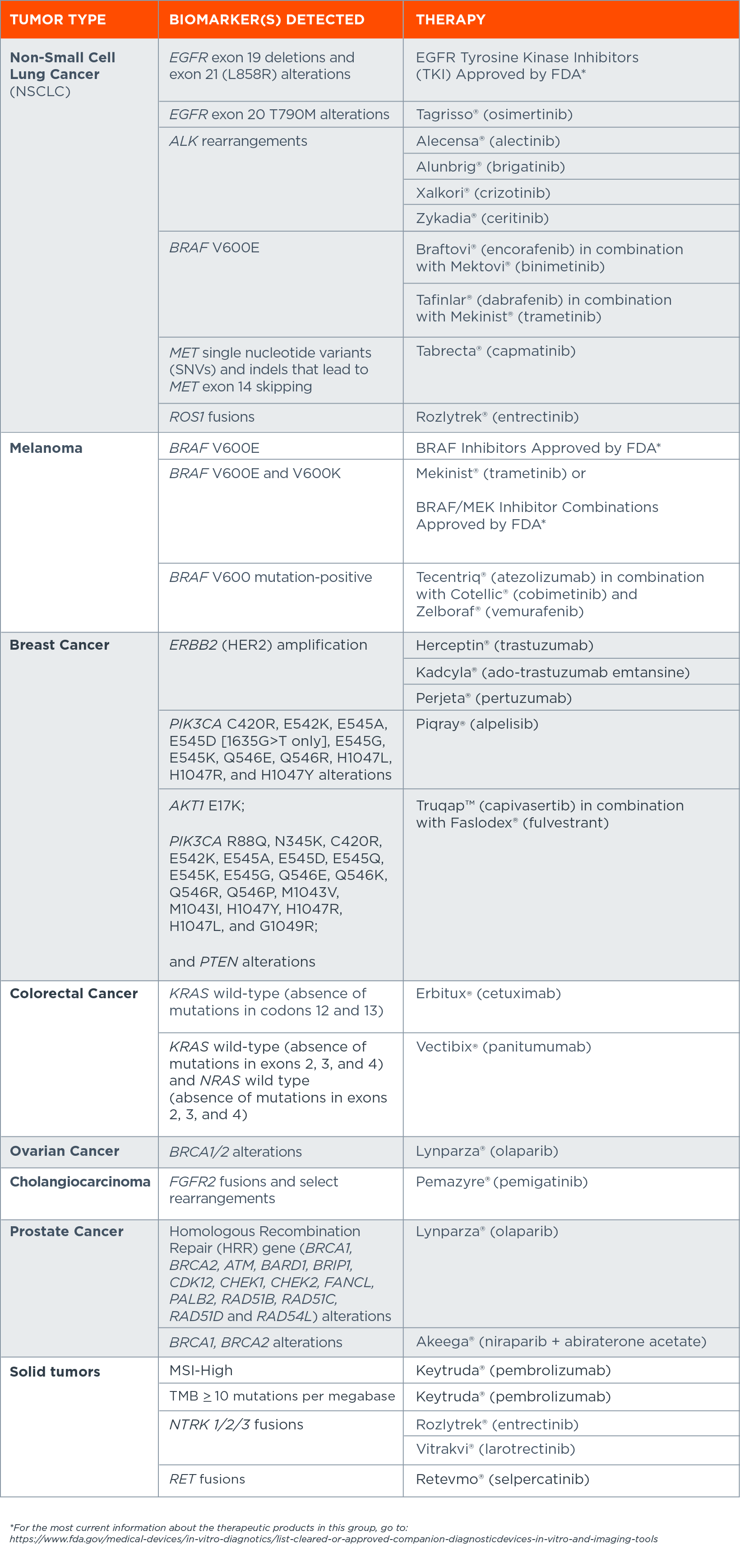
FoundationOne®CDx is a tissue-based comprehensive genomic profiling test with the following FDA-approved companion diagnostic indications, including:[iiii]
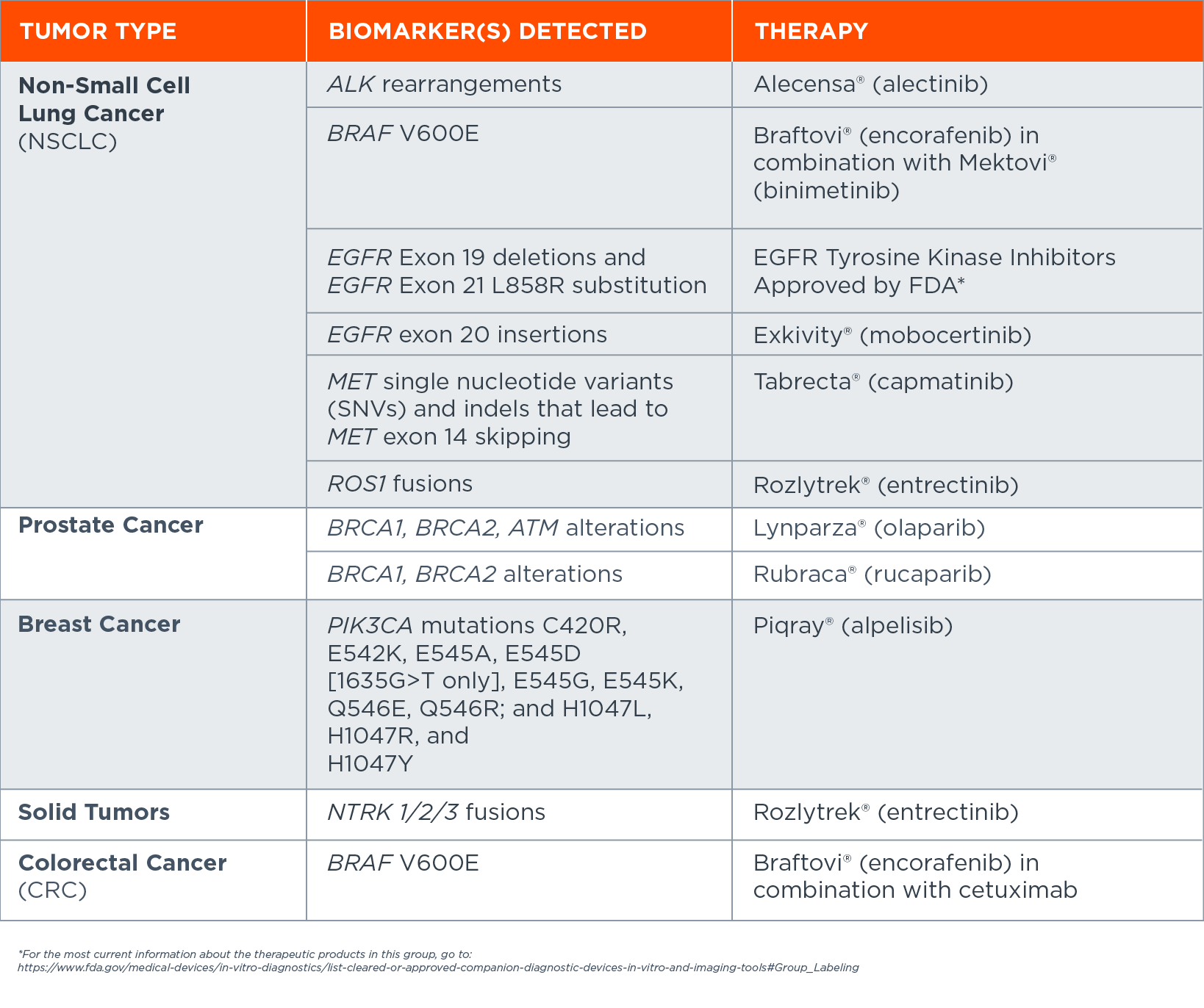
FoundationOne®Liquid CDx is a minimally invasive blood-based comprehensive genomic profiling test with the following FDA-approved companion diagnostic indications, including:
About FoundationOne Liquid CDx
FoundationOne®Liquid CDx is for prescription use only and is a qualitative next-generation sequencing based in vitro diagnostic test for advanced cancer patients with solid tumors. The test analyzes 324 genes utilizing circulating cell-free DNA and is FDA-approved to report short variants in 311 genes and as a companion diagnostic to identify patients who may benefit from treatment with specific therapies (listed in Table 1 of the Intended Use) in accordance with the approved therapeutic product labeling. Additional genomic findings may be reported and are not prescriptive or conclusive for labeled use of any specific therapeutic product. Use of the test does not guarantee a patient will be matched to a treatment. A negative result does not rule out the presence of an alteration. When considering eligibility for certain therapies for which FoundationOne Liquid CDx is a companion diagnostic, testing of plasma is only appropriate where tumor tissue is not available. Patients who are negative for other companion diagnostic mutations should be reflexed to tumor tissue testing and mutation status confirmed using an FDA-approved tumor tissue test, if feasible. For the complete label, including companion diagnostic indications and complete risk information, please visit www.F1LCDxLabel.com.
About FoundationOne CDx
FoundationOne®CDx is a qualitative next-generation sequencing based in vitro diagnostic test for advanced cancer patients with solid tumors and is for prescription use only. The test analyzes 324 genes as well as genomic signatures including microsatellite instability (MSI) and tumor mutational burden (TMB) and is a companion diagnostic to identify patients who may benefit from treatment with specific therapies in accordance with the approved therapeutic product labeling. Additional genomic findings may be reported and are not prescriptive or conclusive for labeled use of any specific therapeutic product. Use of the test does not guarantee a patient will be matched to a treatment. A negative result does not rule out the presence of an alteration. Some patients may require a biopsy. For the complete label, including companion diagnostic indications and important risk information, please visit www.F1CDxLabel.com.
References
[i] “Companion Diagnostics,” US Food and Drug Administration. Available at: https://www.fda.gov/medical-devices/vitro-diagnostics/companion-diagnostics
[ii] Pant et al. Navigating the Rapids: The Development of Regulated Next-Generation Sequencing-Based Clinical Trial Assays and Companion Diagnostics. Frontiers in Oncology. 2014;4:78.
[iii] Clark et al. Analytical validation of a hybrid capture-based next-generation sequencing clinical assay for genomic profiling of cell-free circulating tumor DNA. The Journal of Molecular Diagnostics. 2018. doi: https://doi.org/10.1016/j.jmoldx.2018.05.004
[iiii] List of Cleared or Approved Companion Diagnostic Devices (In Vitro and Imaging Tools), US Food and Drug Administration https://www.fda.gov/medical-devices/vitro-diagnostics/list-cleared-or-approved-companion-diagnostic-devices-vitro-and-imaging-tools