Are your negative liquid biopsy results really negative?
FoundationOne®Liquid CDx reports include ctDNA tumor fraction*** as a professional service which has not been reviewed or approved by the FDA.
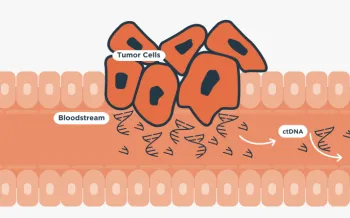
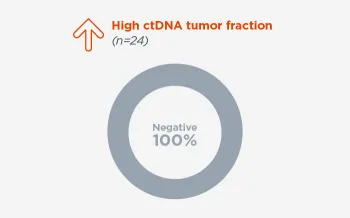
ctDNA tumor fraction is a determination of the amount of circulating tumor DNA as a fraction of total cell free DNA in a blood sample that accounts for aneuploidy, variant allele frequency, fragment length information, clonal hematopoiesis predictions and known tumor-associated alterations.
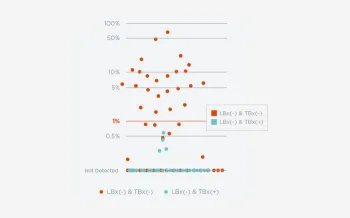
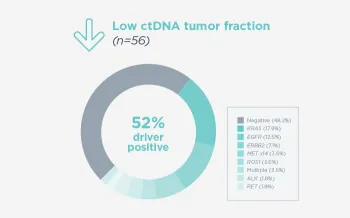
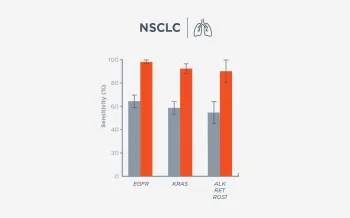
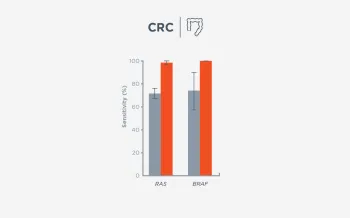
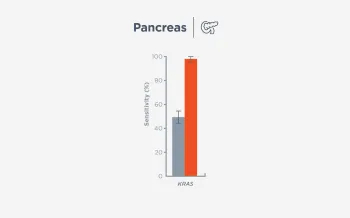
When FoundationOne Liquid CDx ctDNA tumor fraction is low and results are negative, reflexing to tissue may find actionable gene alterations (52% more actionable alterations in NSCLC6).
Next steps in patient care protocols tend to be more clear with positive results. However, when liquid biopsy results are negative, be better informed with ctDNA tumor fraction.